14+ Le Chatelier'S Principle Pdf
So portlandite solubility increases at low temperature. Chemistry also addresses the nature of chemical bonds in.

Using Le Chatelier S Principle To Predict The Result Of Changing Temperature Youtube
Feedback in general is the process in which changing one quantity changes a second quantity and the change in.

. How to fill MET 2023 Application Form. NEET Biology Syllabus 2023. This is the condition of optimal functioning for the organism and includes many variables such as body temperature and fluid balance being kept within certain pre-set limits homeostatic range.
This phenomenon is based on Le Chateliers principle which when extrapolated to exothermic processes states that increasing temperature decreases the magnitude of the exothermic process Brown. ˈ ʃ ɑː t əl j eɪ also called Chateliers principle or the Equilibrium Law is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibriaThe principle is named after French chemist Henry Louis Le Chatelier and sometimes also credited to Karl Ferdinand Braun. Using a lighter and the fumes are inhaled or smoked from glass paraphernalia known as base pipes most commonly colloquially referred to as bowl globe.
Nature employs molecular buffers like HPO 4-and TBG to maintain a constant concentration of active biomolecules using Le Chateliers principle for H and thyroxine respectively. Urea serves an important role in the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of. Three isotopes occur naturally 12 C and 13 C being stable while 14 C is a.
There is an opposition to change the state of an equilibrium reaction. A lowering of temperature favors the removal of dissolution heat from the system and thus favors dissolution of CaOH 2. It is thus the simplest amide of carbamic acid.
This state results when the forward reaction proceeds at the same rate as the reverse reactionThe reaction rates of the. The amount of urate in the body depends on the. This can come from the intrinsic energy of the molecules themselves or.
Equilibrium and Le Chateliers principle. In a chemical reaction chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time so that there is no observable change in the properties of the system. The history of chemistry represents a time span from ancient history to the present.
NCERT Solutions for Class 11 English. Carbon from Latin carbo coal is a chemical element with the symbol C and atomic number 6. Temperature Pressure Concentration Catalyst.
Urea also known as carbamide is an organic compound with chemical formula CONH 2 2This amide has two amino groups NH 2 joined by a carbonyl functional group CO. Join an activity with your class and find or create your own quizzes and flashcards. Hyperuricaemia or hyperuricemia is an abnormally high level of uric acid in the bloodIn the pH conditions of body fluid uric acid exists largely as urate the ion form.
It is a natural science that covers the elements that make up matter to the compounds made of atoms molecules and ions. By 1000 BC civilizations used technologies that would eventually form the basis of the various branches of chemistry. Producing gold coins microscale - student instructions and worksheet Editable handout.
Serum uric acid concentrations greater than 6 mgdL for females 7 mgdL for men and 55 mgdL for youth under 18 years old are defined as hyperuricemia. The electron density plots of the remaining electrons in the second energy level are very different from the spherical s orbitals. Lets Discuss the NEET Syllabus PDF in a detailed manner.
Some of the questions on the student sheet are more suitable for higher-level learners. The practical experiment is suitable for all learners within the 1416 age range group. Handout PDF Size 035 mb.
Crystal methamphetamine most commonly colloquially known as crystal meth or ice and free base forms of amphetamine are sufficiently volatile substances and this allows them to be vaporized by high heat ie. Le Chateliers principle states that the system opposes changes in conditions from equilibrium states ie. NCERT Solutions for Class 11 Chemistry.
Ionic equilibrium- ionization of acids and bases strong and weak electrolytes degree of ionization ionization of polybasic acids acid strength concept of PH Hydrolysis of salts elementary. They are basically in chronological order subject to the uncertainty of multiprocessing. Transforming one structure to another requires the input of energy to cross an energy barrier.
Le Chateliers principle pronounced UK. MET 2023 Registration - The candidates have to register themselves to fill out the application form by using their email-id mobile number and passwordAfter the registration process the candidates will receive the login credential on their Email IDs which they will have to use at the time of the application process to download. Carbon makes up only about 0025 percent of Earths crust.
The dissolution of calcium hydroxide in water is also an exothermic process ΔH 0 and obeys the van t Hoff equation and Le Chateliers principle. The dumbbell has two lobes and between the two lobes there is. Equilibrium constant factors affecting equilibrium - Le Chateliers principle ionic equilibrium- ionization of acids and bases strong and weak electrolytes degree of ionization ionization of poly basic acids acid strength concept of pH hydrolysis of salts.
The chemical equation described in section 41 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sidesThis is a requirement the equation must satisfy to be consistent with the law of conservation of matter. Free PDF download of CBSE Class 11 Chemistry Syllabus as per NCERT guidelines. Students should be able to.
In biology homeostasis British also homoeostasis hɒmɪəʊˈsteɪsɪs is the state of steady internal physical and chemical conditions maintained by living systems. Le Chateliers principle can be used to predict the effects of changes in temperature pressure and concentration on the position of equilibrium in homogeneous reactions. It is nonmetallic and tetravalentits atom making four electrons available to form covalent chemical bondsIt belongs to group 14 of the periodic table.
Use Le Chateliers principle to predict qualitatively the effect of changes in temperature pressure and concentration on the position of. Examples include the discovery of fire extracting metals from ores making pottery and glazes fermenting beer and wine extracting chemicals from plants for medicine and perfume. IB Diploma Chemistry HL Textbook pdf.
Class 12 Physics Book PDF. The electron-density plots for these orbitals take on a shape that is best described as a dumbbell. A slit S is illuminated by a monochromatic source of light to give two coherent sources P1 and P2.
Their composition structure properties behavior and the changes they undergo during a reaction with other substances. Chemistry is the scientific study of the properties and behavior of matter. Climate change feedbacks are important in the understanding of global warming because feedback processes amplify or diminish the effect of each climate forcing and so play an important part in determining the climate sensitivity and future climate state.
Factors affecting equilibrium-Le Chateliers principle. L ə ʃ æ ˈ t ɛ l j eɪ or US.

Download Pdf Idfc

Equilibrium And Le Chatelier S Principle

Women War And Wages The Effect Of Female Labor Supply On The Wage Structure At Midcentury Journal Of Political Economy Vol 112 No 3
Feralis Booster Dat Study Schedule 2021 Feralis Booster Study Schedule Specifically For The 2021 Studocu
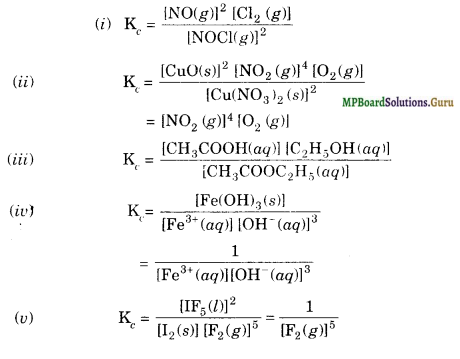
Mp Board Class 11th Chemistry Solutions Chapter 7 Equilibrium Mp Board Solutions

Chemistry Lab 14 Le Chatelier S Principle Pdf Chemical Equilibrium Analytical Chemistry
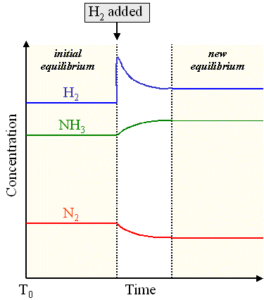
Le Chatelier S Principle Statement Explanation And Examples
Preparing To Study A Level Chemistry Beyond Teacher Made

12th Chemistry Vol2 Em Www Tntextbooks In Pdf Chemistry Notes Teachmint

What Is Le Chatelier S Principle In Chemistry Socratic
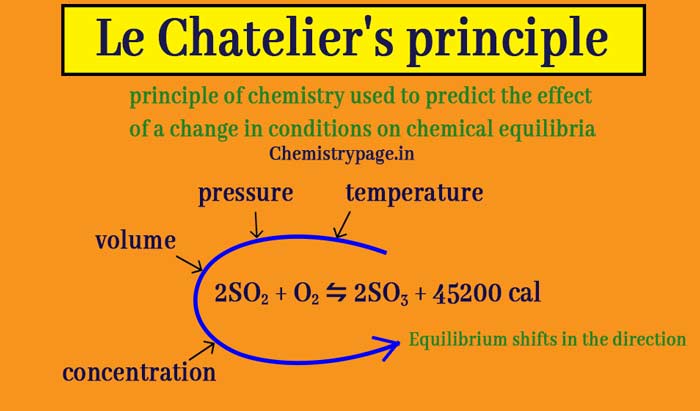
What Is Le Chatelier S Principle In Chemistry Temperature And Pressure Effect Chemistry Page

Pdf Solucionario De Quimica 10 Ed Raymond Chang Alejandra Zamudio Academia Edu

12th Chemistry Vol2 Em Www Tntextbooks In Pdf Chemistry Notes Teachmint
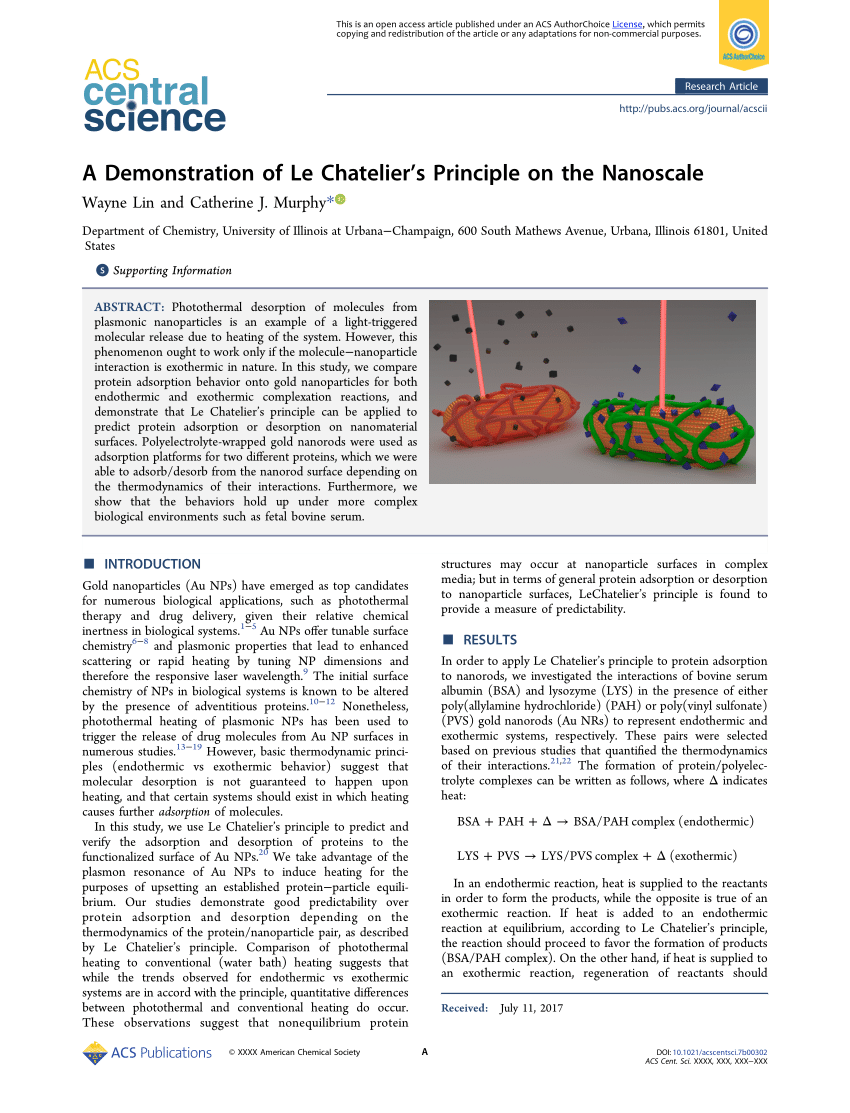
Pdf A Demonstration Of Le Chatelier S Principle On The Nanoscale

Pdf Chemical Fundamentals Geology Gill 2015 Juan Henao Valencia Academia Edu

Aqa Chemistry Revision Combined Higher Mat Bundle Gcse

Pdf Chapter 1 Chemistry The Study Of Change Problem Categories Kim Karl Limpiada Academia Edu